Cevira® – in development for the non-surgical treatment of high-grade squamous intraepithelial lesions, including all HPV sub-types
Cevira® (APL-1702) is a photodynamic drug-device combination product in development. Based on the principles of photodynamic therapy, the Cevira product aims to use a photosensitizer in combination with light activation to produce a therapeutic effect as a non-surgical treatment of high-grade squamous intraepithelial lesions (HSIL), including all human papilloma virus (HPV) sub-types.
For patients with high grade squamous intraepithelial lesions, common surgical procedures (e.g. LEEP, cold knife conization) are associated with increased risk of undesirable side effects including bleeding, infection, scarring (stenosis), infertility and complications in later pregnancies with risk of pre-term delivery.
Cevira is intended to be placed on the cervix by a gynecologist and removed by the patient at the end of treatment, with no disruption to normal daily activities during treatment (see graphic 2 below).

1: Cevira drug delivery device
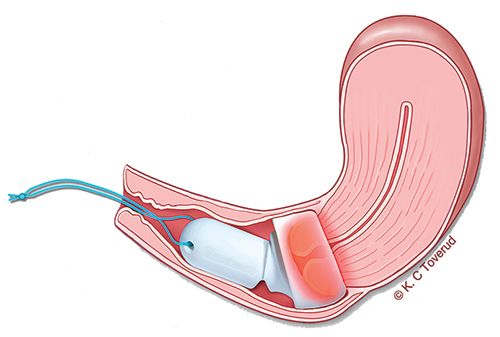
2: Illustration of Cevira inserted on the cervix
Photocure developed Cevira through Phase 1 and Phase 2 trials, and the global rights for development and commercialization were out-licensed to Asieris Meditech Co., Ltd in 2019. Read more
In November 2020 Asieris initiated the phase III clinical trial for APL-1702 (Cevira®), Clinical trial number: NCT04484415.